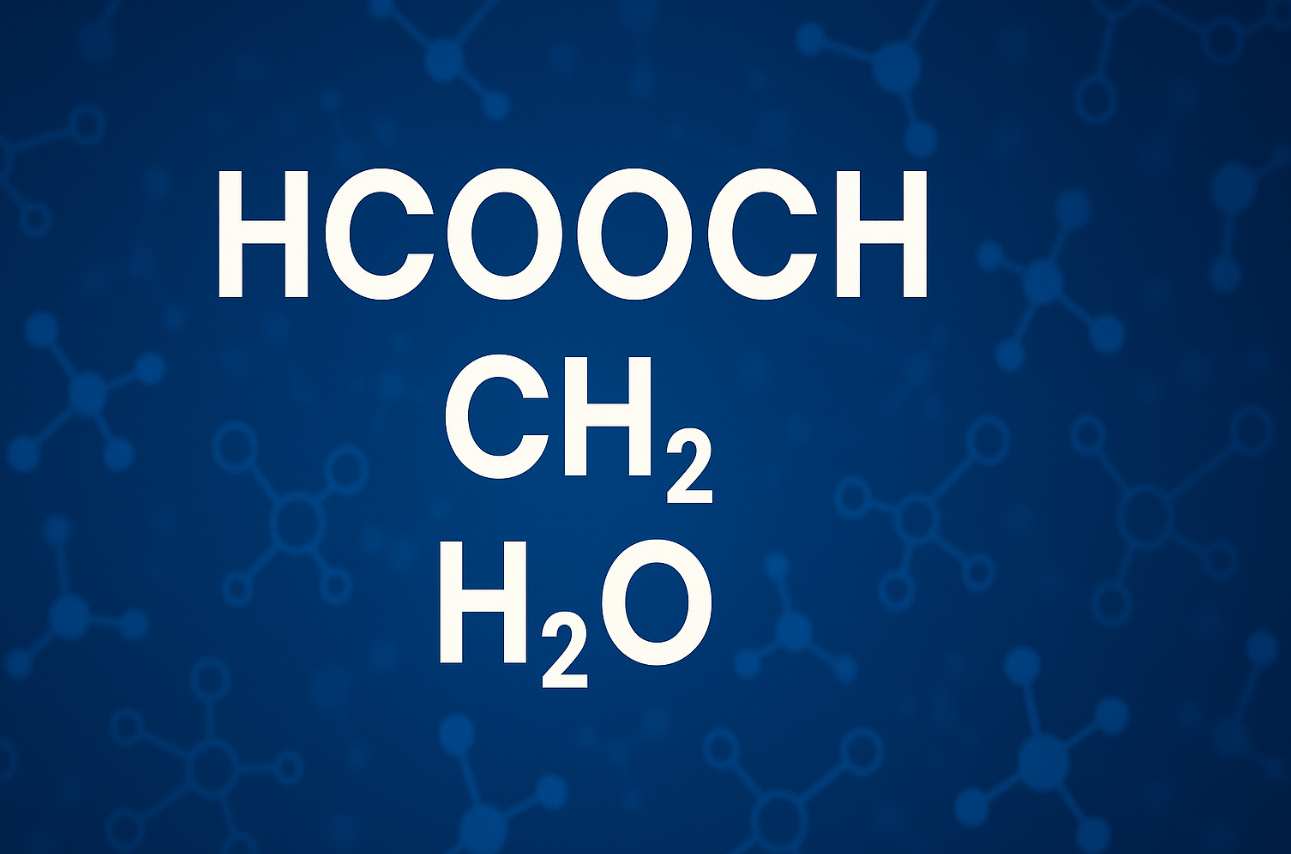
Organic chemistry is a fascinating field that unveils the complexities of molecular interactions. At its core, three molecules stand out: HCOOCH, CH2, and H2O. Each plays a crucial role in various chemical processes and reactions. Understanding their relationships enhances our grasp of organic compounds’ behavior and applications. Join us as we explore this triad’s fundamentals, reactivity patterns, practical uses, safety considerations, and future research directions. This journey promises insights into how these molecules shape industries and scientific advancements.
Fundamentals of the Triad
HCOOCH, CH2, and H2O form a triad that is pivotal in organic chemistry. Each molecule contributes unique properties that facilitate diverse reactions. HCOOCH represents an ester functional group, while CH2 signifies the simplicity of hydrocarbons. Water (H2O) serves as both a solvent and reactant.
Together, these molecules interact dynamically within various chemical contexts. Their roles extend from basic laboratory experiments to extensive industrial processes. Understanding their fundamentals lays the groundwork for exploring complex organic systems effectively.
Key Concepts
Understanding hcooch ch2 h2o offers insights into fundamental organic chemistry concepts. Each molecule plays a vital role in various chemical processes and interactions.
HCOOCH represents formate esters, while CH2 denotes methylene groups. Water (H2O) is essential for many reactions. Together, they create a dynamic environment for studying molecular behavior and reactivity patterns within organic compounds. Their interconnections facilitate diverse applications across scientific fields, making them crucial to both theoretical understanding and practical implementations in chemistry.
Molecular Structure Overview
The molecular structures of HCOOCH, CH2, and H2O are essential in understanding their interactions. HCOOCH, or methyl formate, features a carbonyl group attached to an ether linkage. This configuration contributes to its unique properties.
In contrast, CH2 represents the simplest hydrocarbon unit—methylene. It’s crucial in various organic compounds. Meanwhile, H2O’s polar nature allows for hydrogen bonding with other molecules, significantly influencing reactions involving these substances. The interplay between these structures drives many chemical processes in organic chemistry.
Visualizing Interactions
Visualizing interactions between hcooch ch2 h2o is essential for understanding their chemical behavior. Molecular modeling software can create three-dimensional representations of these molecules, showcasing how they interact at the atomic level.
These visualizations reveal potential hydrogen bonding patterns and steric hindrance effects. By observing these molecular arrangements, researchers can predict reaction pathways and optimize conditions for synthesis or application in various fields like pharmaceuticals or materials science.
Chemical Reactivity
Chemical reactivity is a fundamental aspect of understanding molecules like HCOOCH, CH2, and H2O. Each molecule has distinct properties that determine how they interact with one another. For instance, the functional groups in HCOOCH lead to unique reactions due to potential hydrogen bonding and electronegativity differences.
Reactivity patterns can be observed when these molecules undergo various chemical transformations. Understanding these patterns helps chemists predict outcomes in reactions involving organic compounds and design new synthetic pathways for desired products.
Reactivity Patterns
HCOOCH, CH2, and H2O exhibit distinct reactivity patterns crucial to organic chemistry. The ester functional group in HCOOCH makes it susceptible to nucleophilic attacks, which is critical in various synthesis processes.
Conversely, CH2 groups often act as reactive intermediates. They can participate in numerous reactions due to their ability to donate electrons or form bonds easily. Water serves as both a reactant and product in many chemical transformations, showcasing its versatility within this molecular triad.
Typical Reactions
HCOOCH, CH2, and H2O participate in several typical reactions that highlight their chemical versatility. These molecules often undergo esterification, where carboxylic acids react with alcohol to form esters and water. This process is essential in producing fragrances and flavors.
Another common reaction involves hydrolysis, where esters break down into acids and alcohols when exposed to water. This reaction is vital in various biological processes and industrial applications, showcasing the importance of these compounds in organic chemistry.
Industrial Applications
HCOOCH, CH2, and H2O play pivotal roles in various industrial sectors. These molecules are essential in the production of solvents, adhesives, and plastics. Their unique properties make them versatile for countless applications.
In pharmaceuticals, they aid in drug formulation and synthesis. Additionally, their interactions contribute to advancements in agrochemicals. The efficiency of these compounds drives innovation across industries while enhancing product performance and sustainability.
Practical Applications
HCOOCH, CH2, and H2O play crucial roles in various fields. In laboratories, they are essential for synthesizing organic compounds and studying reaction pathways. Their unique properties facilitate advancements in research.
In industry, these molecules find applications in the production of pharmaceuticals, plastics, and solvents. They contribute to processes that improve efficiency while reducing waste. Understanding their practical uses drives innovation across multiple sectors, enhancing products that benefit everyday life.
Laboratory Techniques
Laboratory techniques for studying HCOOCH, CH2, and H2O are essential in organic chemistry. Standard methods include spectroscopy, chromatography, and titration. These approaches allow scientists to analyze molecular interactions effectively.
Spectroscopy helps identify functional groups by measuring light absorption or emission. Chromatography separates components based on their affinities for stationary and mobile phases. Titration quantifies concentrations of reactants or products in a solution. Each technique plays a crucial role in understanding the behavior of these molecules in various environments.
Industrial and Real-life Applications
HCOOCH, CH2, and H2O play pivotal roles in various industrial processes. In the production of biodegradable plastics, these molecules serve as building blocks that promote sustainability. Their chemical interactions enhance material properties and reduce environmental impact.
In pharmaceuticals, this triad aids in drug synthesis and development. The unique reactivity patterns of these compounds facilitate complex reactions that yield effective medications while minimizing harmful byproducts. These applications highlight their significance beyond theoretical chemistry into everyday life.
Synthesis and Production Methods
HCOOCH, CH2, and H2O synthesis involves various methods tailored to specific reactions. Common approaches include esterification for HCOOCH production and reduction processes that transform organic compounds into simpler molecules like CH2.
For large-scale production, catalytic techniques are often employed. These methods enhance efficiency while minimizing waste. Additionally, green chemistry principles guide the development of sustainable practices in synthesizing these vital molecules, ensuring environmental safety alongside productivity in industrial settings.
Safety and Environmental Considerations
Proper handling of HCOOCH, CH2, and H2O is essential to ensure safety in the laboratory and industrial settings. Personal protective equipment should always be worn when working with these chemicals to minimize exposure risks.
Environmental benefits can arise from using these molecules responsibly. They offer cleaner alternatives in many reactions. However, awareness of potential hazards remains crucial for minimizing accidents and ensuring a sustainable approach to their use in various applications.
Handling Precautions
When working with HCOOCH, CH2, and H2O, proper handling is critical. Always wear appropriate personal protective equipment (PPE), including gloves and goggles. Ensure that you are in a well-ventilated area to minimize inhalation risks.
Store these substances in labeled containers away from incompatible materials. Follow safety data sheets (SDS) for specific guidelines on handling procedures. Being aware of potential hazards can significantly reduce the risk of accidents during experiments or industrial applications.
Environmental Benefits
The combination of HCOOCH, CH2, and H2O plays a significant role in environmental sustainability. These molecules contribute to processes that help reduce greenhouse gas emissions. For example, they are involved in creating biofuels that can replace fossil fuels.
Additionally, these compounds aid in water purification techniques. They facilitate the breakdown of pollutants and enhance the overall quality of water resources. Utilizing these molecules promotes cleaner air and healthier ecosystems while supporting green chemistry initiatives.
Mitigating Risks
Mitigating risks associated with HCOOCH, CH2, and H2O involves implementing stringent safety protocols. Proper training for personnel handling these substances is essential to prevent accidents. Regular risk assessments can help identify potential hazards in the laboratory or industrial settings.
Additionally, utilizing appropriate personal protective equipment (PPE) reduces exposure risks significantly. Implementing proper storage methods ensures that these molecules are kept safely away from incompatible substances, minimizing the risk of chemical reactions that could lead to dangerous situations.
Future Research and Innovations
Emerging trends in organic chemistry focus on enhancing the efficiency and sustainability of reactions involving HCOOCH, CH2, and H2O. Researchers are exploring advanced catalysts and greener solvents to minimize waste and energy consumption in chemical processes.
Future directions include leveraging biotechnology for more sustainable synthesis methods. Innovations may also involve integrating artificial intelligence to predict molecular interactions better, paving the way for novel applications across various industries while promoting environmental safety.
Emerging Trends
Emerging trends in the study of HCOOCH, CH2, and H2O highlight innovative ways to utilize these molecules. Researchers are focusing on green chemistry practices that minimize environmental impact while maximizing efficiency. This includes developing bio-based feedstocks for organic synthesis.
Additionally, advancements in computational modeling are helping scientists predict molecular interactions more accurately. These approaches enhance our understanding of reactivity patterns and enable tailored applications across various industries, from pharmaceuticals to sustainable materials development.
Science of the Future
The future of molecular science is bright, with HCOOCH, CH2, and H2O leading the charge. Researchers are exploring new synthesis methods that enhance efficiency and reduce waste. This promise opens doors for cleaner production techniques in various industries.
Innovations in catalysis also play a role in understanding these molecules’ interactions. Advanced imaging technologies reveal real-time processes at the molecular level, paving the way for breakthroughs. These developments could redefine chemical manufacturing and environmental sustainability efforts worldwide.
Future Directions in Research
Research on HCOOCH, CH2, and H2O is rapidly advancing. Scientists are exploring new synthetic pathways to enhance efficiency and yield in organic reactions. These studies focus on optimizing conditions for better reactivity.
Moreover, the integration of these molecules in green chemistry initiatives is gaining momentum. Researchers aim to reduce waste and environmental impact while developing sustainable methods for industrial applications. This direction not only addresses current challenges but also paves the way for breakthroughs in various fields within organic chemistry.
Conclusion
The interplay of HCOOCH, CH2, and H2O showcases the fascinating complexity of organic chemistry. Their molecular structures define their unique properties and critical roles in various chemical reactions. Understanding these interactions not only enhances our grasp of fundamental science but also opens doors to innovative applications across industries.
From laboratory techniques to industrial uses, the triad has proven invaluable in synthesis and production processes. Safety considerations are paramount as we navigate potential risks associated with handling these substances. Yet, their environmental benefits cannot be overlooked.
As research continues to evolve, emerging trends promise exciting advancements in this field. Future studies will likely explore novel applications and methods that can further harness the potential of HCOOCH, CH2, and H2O for sustainable development. The journey into this trio’s chemistry is just beginning; discoveries await on the horizon.